
Injectable Water: Meaning, Medical Role, Safety, and How Sterile Water Products Are Evaluated
Injectable Water is a term that people may come across in hospitals, medical facilities, or on medical supply websites. The term may seem straightforward, but in healthcare, it refers to a very tightly controlled category of water of a pharmaceutical-grade that is used as a sterile vehicle for drugs or procedures. Apart from regular drinking water or even purified water that is meant for household use, the water that is of an injectable-grade has to meet the strictest standards.
This article represents the definition of Injectable Water in precise scientific terms: the composition of the water, the equipment used for the production, and the medical environments for its application. It also elaborates on the warnings and cautionary details that come with the product and differ it from the related concepts such as bacteriostatic water.
What “Injectable Water” Means in Scientific and Clinical Terms
In clinical language, the term “Injectable Water” most of the time is understood as “Sterile Water for Injection” (commonly abbreviated as SWFI) or “Water for Injection” (WFI). WFI is a pharmacopoeia standard term for ultrapure water that is produced specifically for the manufacture of medicinal products that are given parenterally, i.e., administered in the body through the routes that bypass the digestive system.
Sterile Water for Injection is basically one that has gone through the process of sterilization and is intended for medical use. It does not have antimicrobial agents, buffers, or any other solutes added, and it is available in single-dose containers or bulk packaging for pharmacies.

Where Injectable Water Is Used in Medical Environments
Hospitals and licensed compounding facilities use Injectable Water to a few controlled purposes. Common clinical uses include:
Reconstitution or dilution of medications
Many injectable drugs come as powders or concentrated solutions. Sterile Water for Injection may be utilized to dissolve or dilute these drugs just before administration, and it is a must to follow the drug’s official prescribing directions strictly.
Pharmacy compounding
Pharmacy bulk packages of sterile water are sources of sterile vehicles during the aseptic preparation of intravenous admixtures. They are only intended for use in pharmacy clean environments with strict handling rules.
Biologics and laboratory applications
Water for Injection is among the materials used in the preparation of culture media or the rinsing of equipment in pharmaceutical manufacturing. These uses necessitate the same purity and endotoxin controls as they affect patient-facing products.
Safety Considerations and Why Plain Sterile Water Can Be Dangerous If Misused
One of the very essential things about Injected Water is that it is a hypotonic solution. Blood and body fluids of a human being are generally isotonic, that is they have dissolved salts and substances at a stable concentration. A simple sterile water is a solution with no solutes. So, if it is directly introduced into the blood plasma, it can make red blood cells swell and eventually burst.
Safety summary
- Never use plain sterile water directly as an infusion. It should be mixed with a suitable solute first to obtain a physiologically compatible solution.
- Do not consider sterile water as “safe to inject by itself”. Sterility does not mean that the product is physiologically compatible.
- Manage your risks properly. After a container is opened, the risk of contamination goes up very fast. Large containers are meant for professional pharmacy conditions and should not be kept for repeated informal uses.
These statements are present because injuries have been caused due to the mistaking of sterile water packaging for intravenous fluid or its use in a different manner than that indicated on the label.
Regulatory Distinctions: Sterile Water, Water for Injection, and Bacteriostatic Water
People see multiple similar terms online. Understanding the regulatory differences helps avoid confusion.
Comparison table
| Product term | What it is (regulatory/clinical meaning) | Contains preservatives? | Typical clinical role | Key safety note |
| Water for Injection (WFI) | High-purity compendial water used to manufacture injectable medicines | No | Manufacturing excipient | Must meet endotoxin and purity limits |
| Sterile Water for Injection (SWFI) | Sterilized WFI packaged as a diluent for injectable drugs | No | Reconstitution/dilution in aseptic prep | Hypotonic; not for direct infusion |
| Bacteriostatic Water | Sterile water with a bacteriostatic agent (commonly benzyl alcohol) to inhibit microbial growth after opening | Yes | Multi-dose reconstitution when specifically indicated | Not interchangeable with SWFI; preservative can be unsuitable for some patients |
Why this distinction matters
- Interchangeability cannot be assumed. A drug label indicates the approved diluent.
- Preservatives alter the risk profile. Bacteriostatic products are multi-dose by the nature of their design, whereas classic SWFI is generally single-dose.
- Label indication has legal significance. “For Injection,” “For Irrigation,” and “For Inhalation” are different categories with different clinical use.
How Consumers Evaluate Sterile/Injectable Water Products Online (Without Encouraging Use)
Although, injectable water is a medical supply, people still come across it in marketplace listings or third-party distributor pages. An unbiased manner of considering the evaluation of the internet would be to search for legality and conformity indications, rather than “best use” instructions.
Clear pharmacopoeial or regulatory standard
Generally, a proper SWFI would be indicated to a recognized standard such as USP or an equivalent national pharmacopeia.
Explicit warning language – Injectable Water
Good labels make it very clear that the product is not isotonic and that it should not be given directly without the addition of other substances.
Manufacturer identity and lot/expiry details
Fluids used in medicine under regulation have features that can be traced like batch numbers and expiration dates.
Red flags in online contexts – Injectable Water
- Vague wording like “injectable-grade water” without any standard listed.
- Missing safety warnings despite being labeled for injection.
- No manufacturer, no lot number, or no expiry.
- Promotional claims tied to self-use, cosmetic injection, bodybuilding, or “DIY” mixing.
These are outside legitimate medical labeling and often unsafe.
A simple visual guide: risk rises as clinical signals disappear
Risk level by listing quality (conceptual):
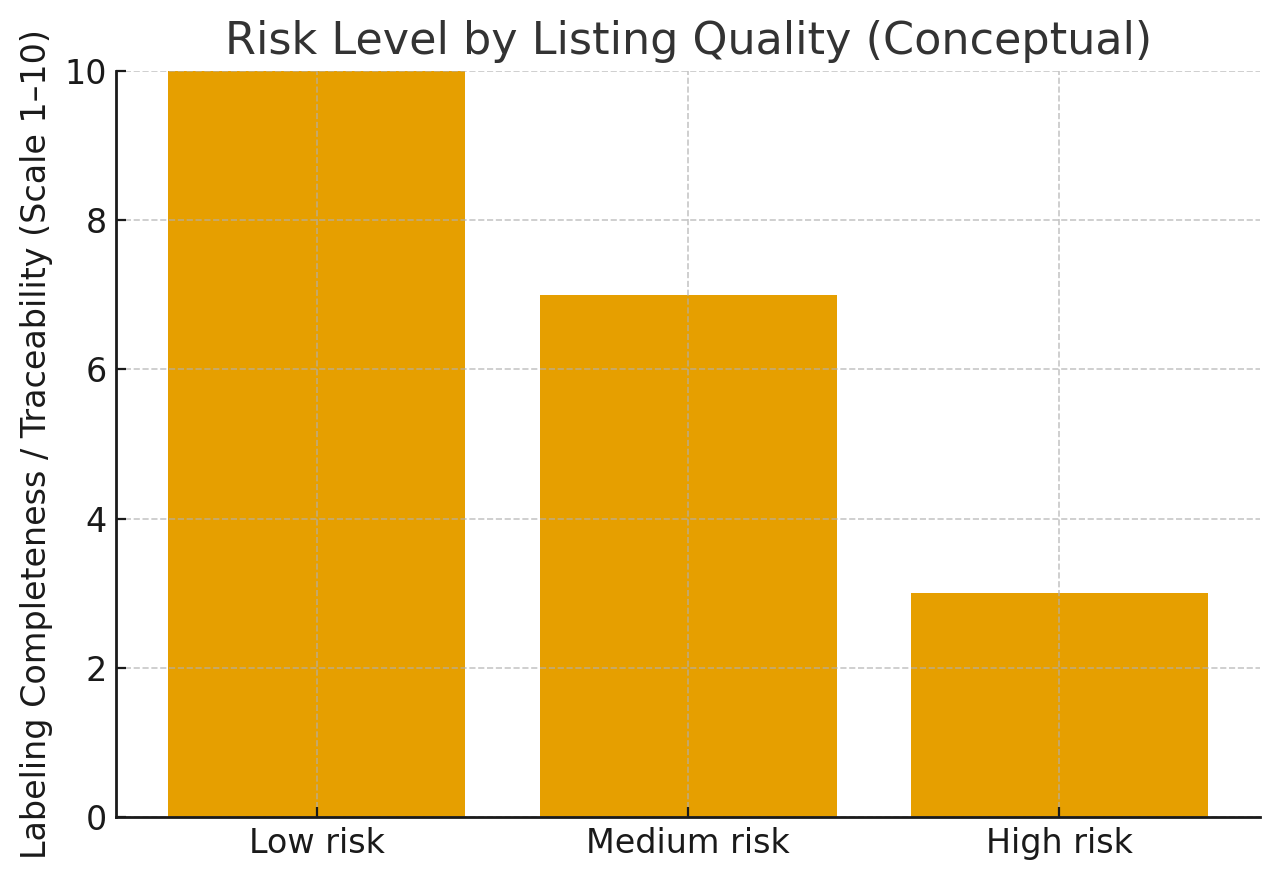
- Low risk is the category with the highest labeling completeness score, which essentially means that it has strong traceability.
- Medium risk is somewhat halfway, as it demonstrates partial but incomplete labeling.
- High risk is at the bottom of the scale, thus it is indicative of a weak or missing standards/warnings.
- General trend: improved listing quality is associated with a lower risk level.
This isn’t a buying recommendation—just a way to understand why regulators focus on labeling completeness.
Final Takeaway – Injectable Water
Injectable Water is not “just water.” In medical terms of Trutan, it is a reference to water that is free of any infection, irritants, and of high pharmaceutical-grade used as a vehicle under strict control for the preparation of injectable therapies. It should comply with pharmacopeial purity standards, be marketed in sterile containers, and be aseptically handled.
The quite important safety point to which all the people should pay attention is that a plain sterile water is a hypotonic solution and, as such, it is able to cause a serious injury if it is introduced directly into the bloodstream without a proper solute adjustment made by the professionals.


This Post Has 0 Comments